Amazing Info About How To Find Out Valence Electrons
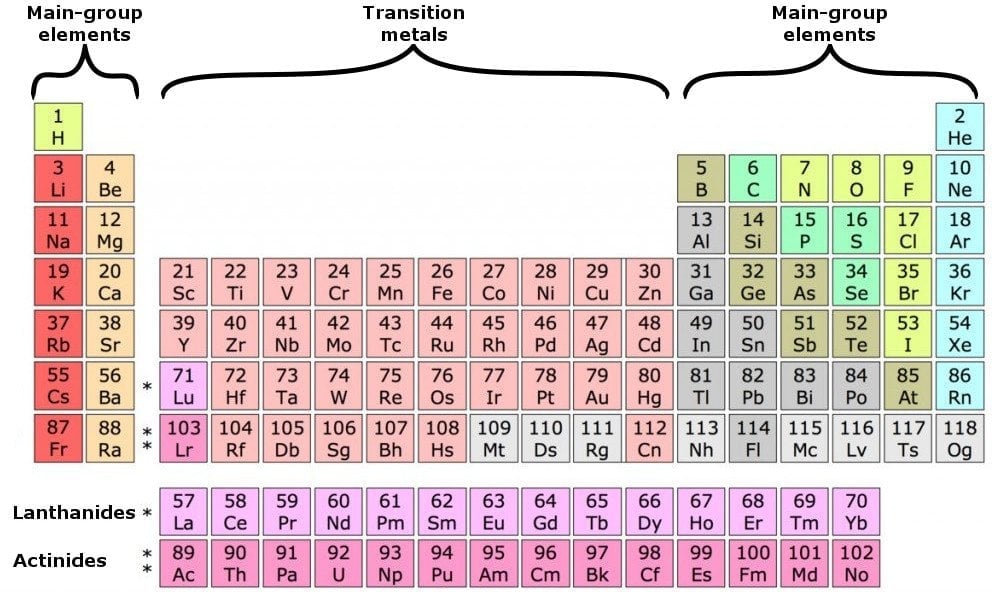
Find a periodic table of elements.
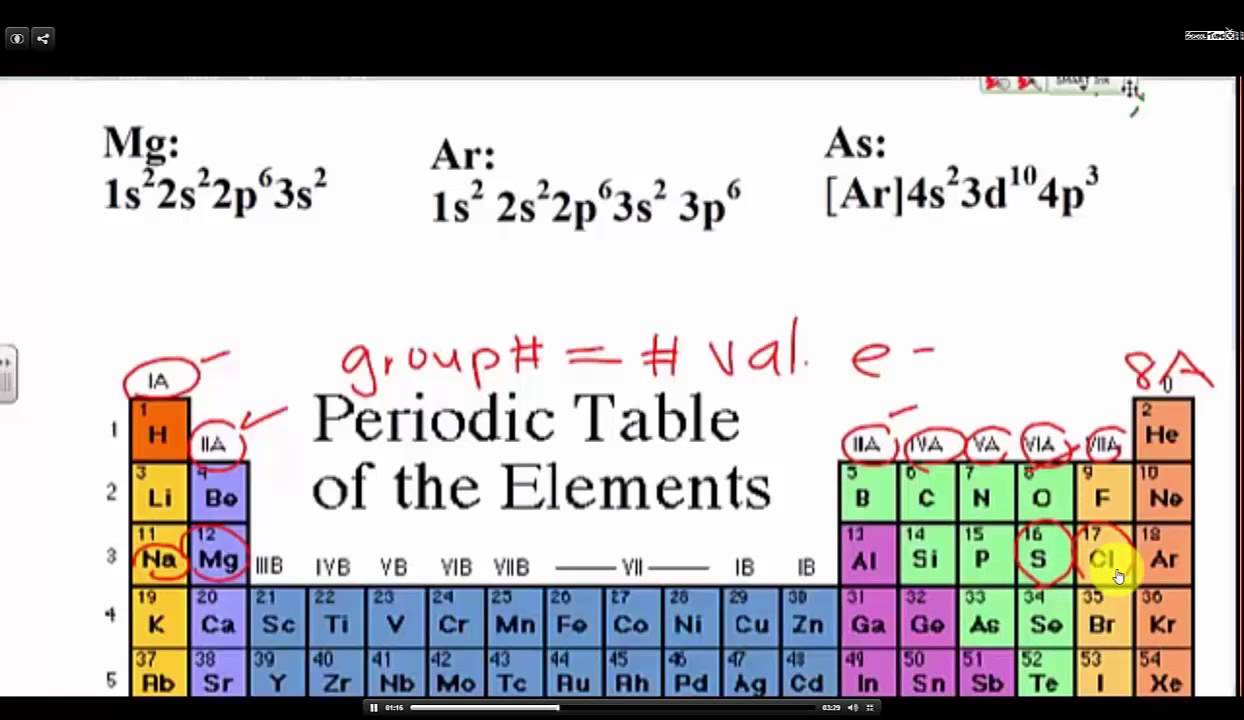
How to find out valence electrons. You can find valence electrons with a shortcut using the periodic table, but it’s good to only do that after you understand why the shortcut works, and to do that you have to. More specifically, you have to see the group wise position of lithium. In order to find the valence electrons of tellurium atom (te), you can use two methods.
From the periodic table to find out the valence electrons of tellurium,. This will be how many valence electrons there are. More specifically, you have to see the group wise position of.
Locate the element on the periodic table. Looking at the periodic table, atoms have a regularly occurring number of valence electrons based on their group number. You are wondering about the question what are valence electrons used for by an element but currently there is no answer, so let kienthuctudonghoa.com summarize and list the top articles.
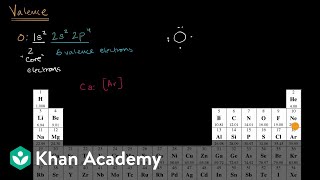
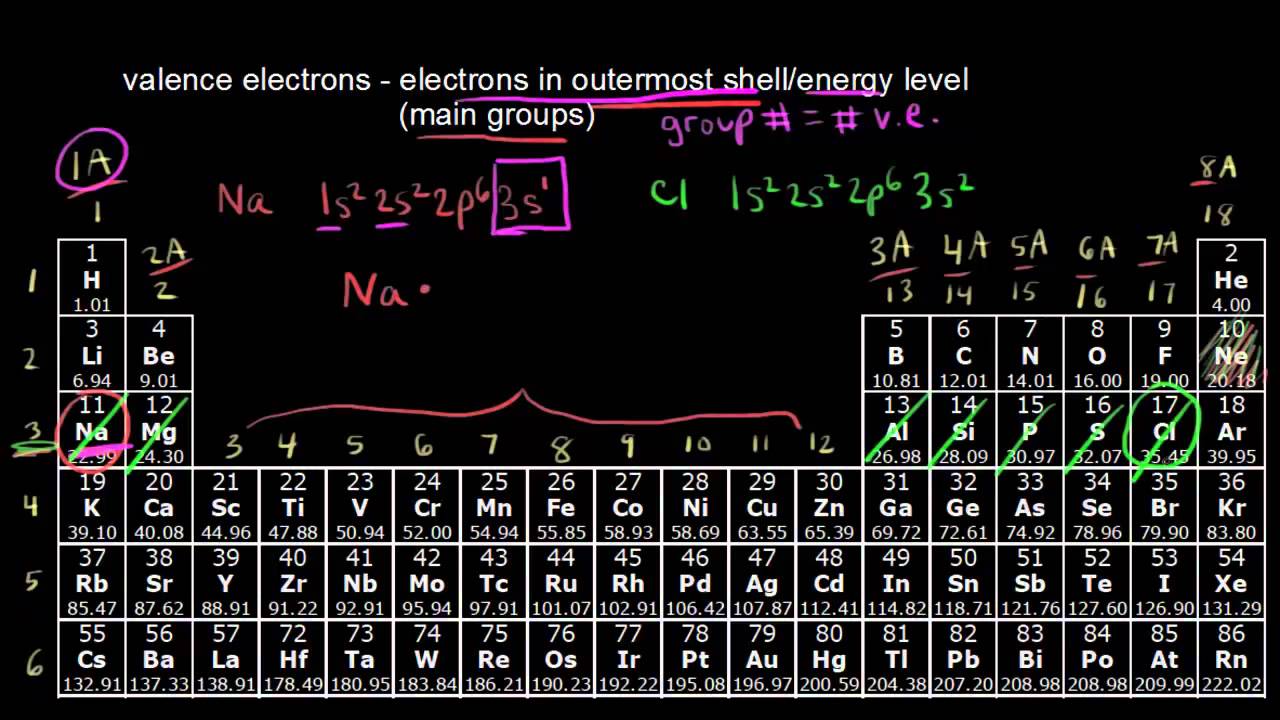
Valence electrons are the electrons in the outermost shell, or energy level, of an atom. If an element is not a transition metal, then valence electrons increase in number as you count. Apply the rule of the periodic table to your element.
More specifically, you have to see the group wise position of. For neutral atoms, the number of valence electrons is equal to the atom’s main group number. In order to find the valence electrons of an iodine atom (i), you can use two methods.
In the case of nitrogen, the highest value of n is 2. To find out the valence electrons of selenium, you have to see the position of selenium in the periodic table. So, in n= 2, you.


![How To Determine The Number Of Valence Electrons In An Element, Ion, Or Molecule [Quick And Easy] - Youtube](https://i.ytimg.com/vi/GEnqFx8MQ5w/maxresdefault.jpg)