Unbelievable Tips About How To Draw Vsepr Diagrams
How many valence electrons does each atom have?;
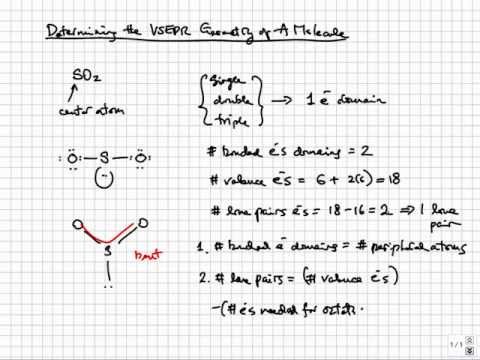
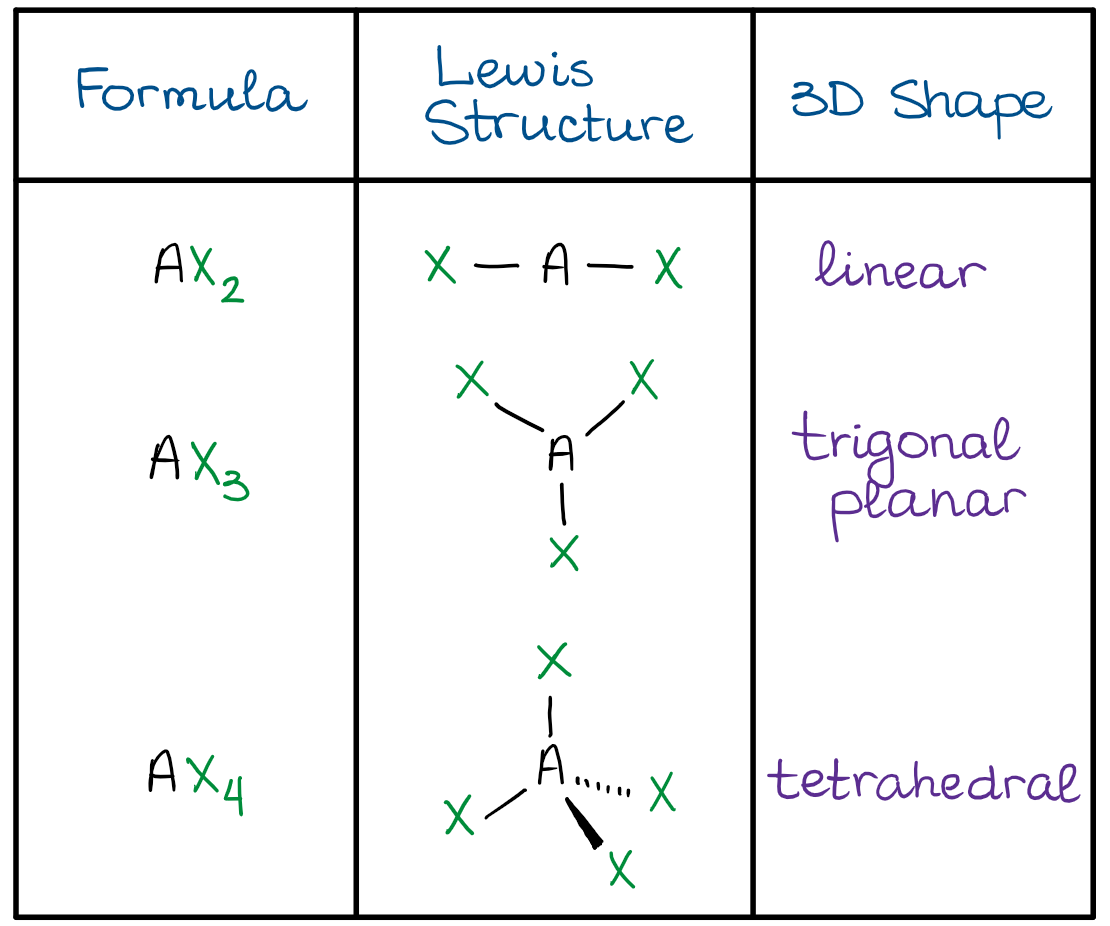
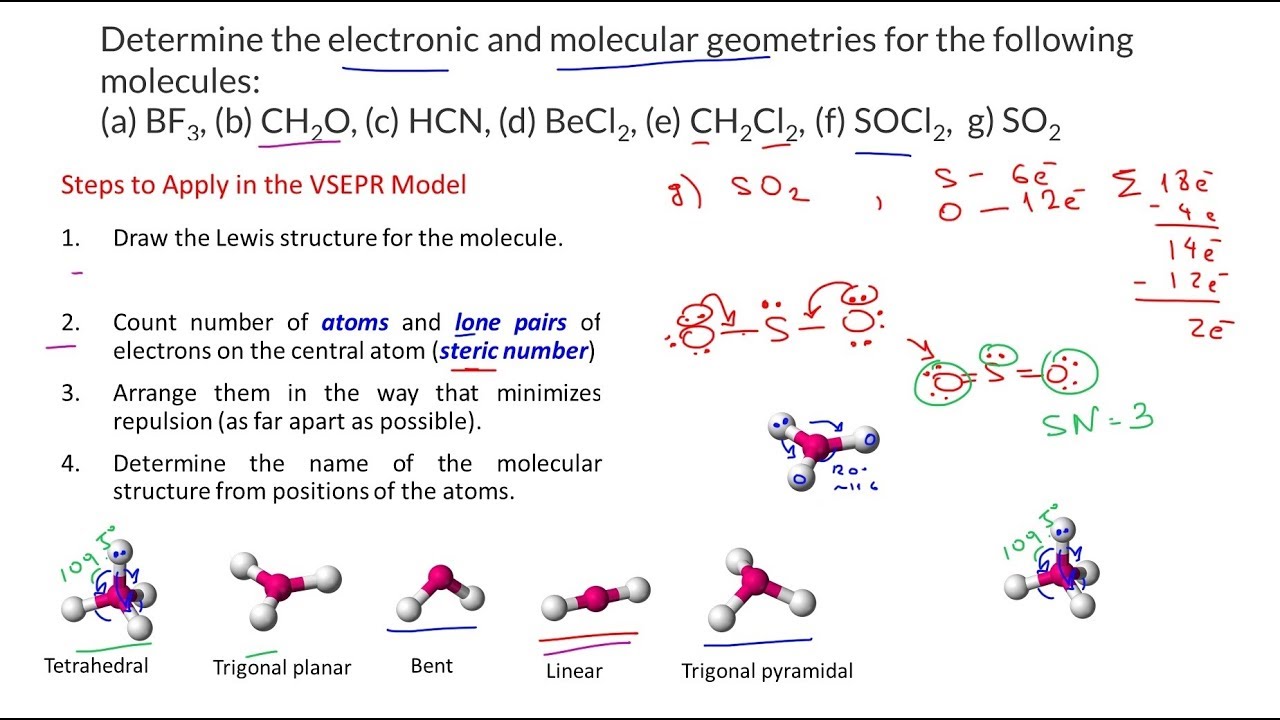
How to draw vsepr diagrams. Lewis diagrams and vsepr structures. Organic chemistry deals mostly with carbon and hydrogens, also called hydrocarbons, but those groups which replace hydrogen and bonds with. The general concept is that the pairs of electrons repel each.
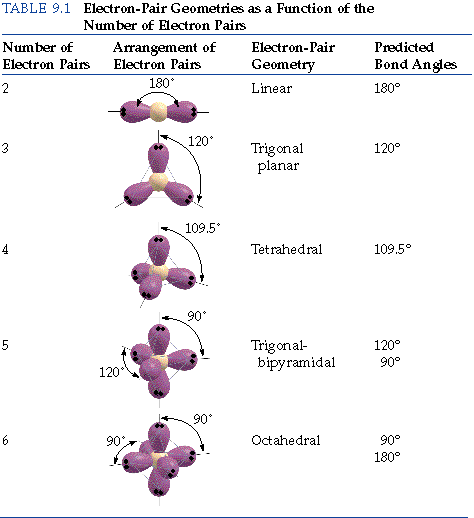
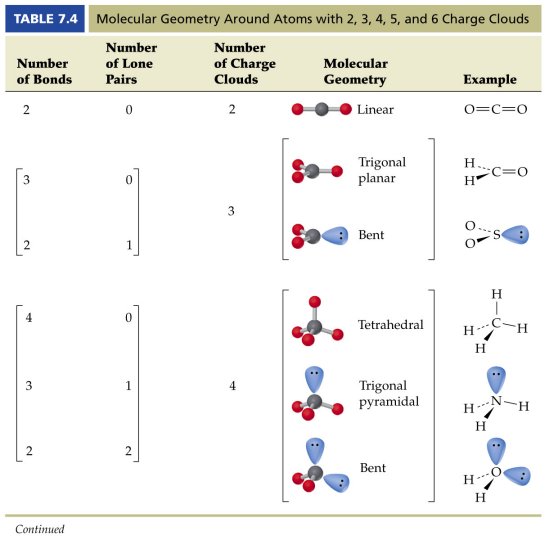
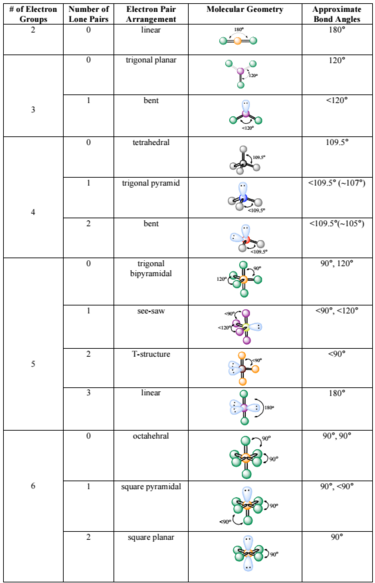
The valence shell electron pair repulsion theory (vsepr) helps us to understand the 3d. Alternate (o 3 r.h.s.) is permitted. The vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence shell of that atom.
The general concept is that the pairs of electrons repel each. The valence shell electron pair repulsion theory (vsepr) helps us to understand the 3d structure of molecules. The valence shell electron pair repulsion theory (vsepr) helps us to understand the 3d structure of molecules.
The general concept is that the pairs of electrons repel each other and. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators. The valence shell electron pair repulsion theory (vsepr) helps us to understand the 3d structure of molecules.
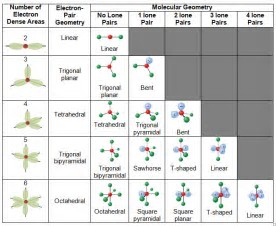
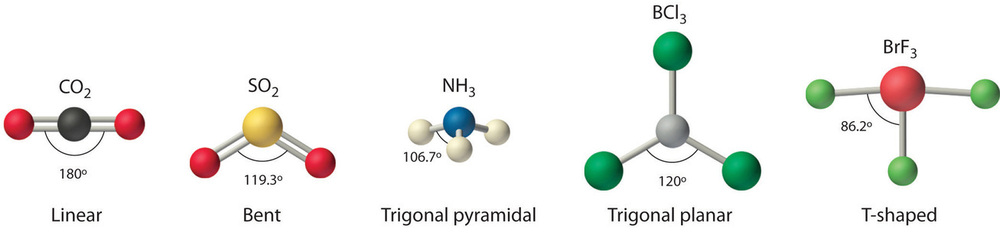
How to draw vsepr diagram for square pyramidal molecule xef4 The number of valence shell electron pairs (bonded and non bonded) around the central atom determine the shape of. About press copyright contact us creators advertise developers terms privacy policy & safety how youtube works test new features press copyright contact us creators.
How do you draw vsepr diagrams? The valence shell electron pair repulsion theory (vsepr) helps us to understand the 3d structure of molecules. E and other “xe” structure the vsepr diagram is best drawn with the position of the “invisible” lone pairs shown (o3 middle);